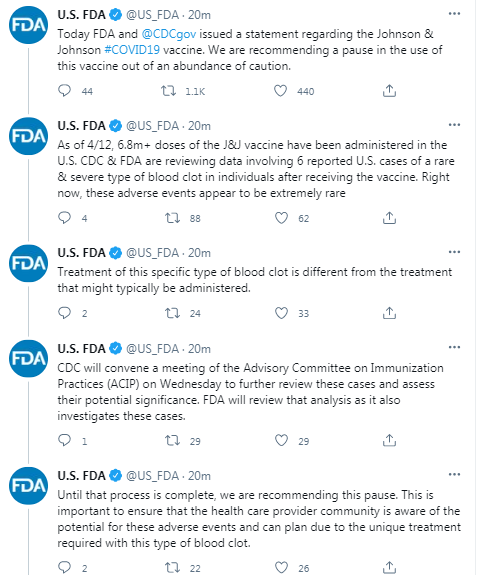
- U.S. FDA recommends pause in use of Johnson & Johnson (JNJ) vaccine after 6 reported U.S. cases of rare and severe type of blood clot in individuals
Market news
Market Focus
Open Demo Account & Personal Page